How Metals are Made
 Do you have a ring on your finger? Is it made from gold, silver, platinum, or another natural metal? Then ponder this: The metal in that ring on your finger is older than the planet you’re standing on.
Do you have a ring on your finger? Is it made from gold, silver, platinum, or another natural metal? Then ponder this: The metal in that ring on your finger is older than the planet you’re standing on.
WHAT IS “METAL”?
Scientifically speaking, metals are naturally occurring chemical elements that are typically hard, lustrous, and good conductors of both heat and electricity. Examples include iron, gold, silver, copper, zinc, nickel, etc., but also elements we don’t normally think of as metals. One is sodium—a metal we regularly eat: Sodium is a soft, silvery white metal that commonly bonds with the element chlorine to form sodium chloride, or common salt.
Another is astatine, which was discovered in 1940 in a lab, where it was created artificially. It wasn’t discovered in nature until 1943. Astatine is highly radioactive, and only a single ounce of it is believed to exist—in total—on Earth. Of the 118 known chemical elements in existence, 88 of them are metals.
REAL ALCHEMY
So, where did all these metals come from? Here’s a very simplified explanation:
All elements, including metals, are made of the same stuff: atomic material—electrons, neutrons, and protons. Atoms of different elements can be distinguished from one another by the number of protons they contain. (The number of neutrons and electrons can vary even among atoms of the same element.) For example, a hydrogen atom contains just one proton. A gold atom has 79. This is true of every one of the countless hydrogen and gold atoms in the universe.
If you could find a way to mash 79 hydrogen atoms together into one atom, you’d have an atom with 79 protons, and therefore you’d have a gold atom. And that’s almost exactly what happens… except it happens inside stars.
THERE’S GOLD IN THEM THAR STARS
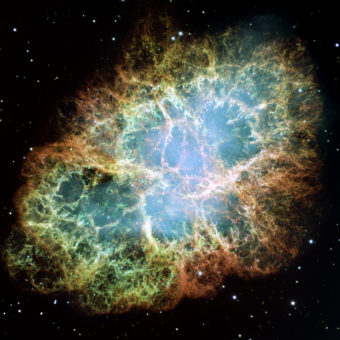
Roughly 13.7 billion years ago, matter first appeared in the form of atoms of the two lightest elements: hydrogen, with one proton, and helium, with two. They remain, by far, the most abundant elements in the universe.
After many millions of years, those first hydrogen and helium atoms collected in clouds of dust and gases so huge they would have to be measured in light years (1 light year = 6 trillion miles or 9.5 trillion kilometers). The clouds eventually gave in to their own enormous gravity and collapsed, forming the first stars. And stars were atom destroyers—hot enough to break down those hydrogen and helium atoms, and fuse the bits back together, remaking them into larger atoms of different, heavier elements.
For example, if you fuse two hydrogen atoms together, you have an atom with two protons—or helium. Fuse three hydrogens together and you get an atom with three protons—lithium, the first and lightest metal. Fuse three heliums together and you get an atom with six protons—carbon. This is what’s happening in all the stars you see in the sky at night. In the massive ones the process can result in the production of heavier and heavier elements, including metals such as titanium (22 protons), and iron (26 protons). If they’re especially massive, they can produce the heaviest metals, such as gold (79 protons), and uranium (92 protons). This is one of the things stars do, and that’s how all the elements—including all those shiny metals—are formed in nature.
Now, how did they get here?
DOWN TO EARTH
In the first few billion years after the Big Bang, billions and billions of stars were born, in the way we just described. Many were extremely massive (hundreds of times larger than our sun) and massive stars live relatively short lives—just a few million years in some cases (smaller stars can live for billions of years)—and then die by exploding as supernovas.
And when those massive stars exploded billions of years ago, they expelled the heavy elements they had been creating, sending them into space. They had, to put it one way, “seeded” the universe with elements, including metals. And super-massive, impossible-to-comprehend amounts of it—trillions and trillions and trillions of megatons of it. That means that when new stars were later formed—they had already been “seeded” with metals left behind by those supernovas.
One of those later, metal-rich stars was our own sun. A quick look at that story:
- About 4.5 billion years ago, a massive cosmic cloud of dust and gas, seeded with lots of heavier elements, collapsed, beginning the process of forming a new star.
- Most of the hydrogen and helium in the cloud became part of the newly formed star. The rest of the dust and gas, including the metals, accumulated in a molten mass, spinning around the new star. The spinning motion flattened out the mass (picture spinning pizza dough) into a molten, spinning disk.
- Over millions of years, as the disk cooled, bits of it clumped together here and there, and those clumps became the planets in our solar system. And the metals in the dust? They became all the metals found in all the planets, including our own.
Our Share: Earth has a lot of metal. Nearly a third of the planet’s mass is the element iron, most of that located in the planet’s core. Another 14 percent is magnesium, 1.5 percent is nickel, and 1.4 percent is aluminum. That’s 49 percent of the planet. The rest of Earth’s metals, including “precious” metals such as gold, silver, platinum, and palladium, exist only in trace amounts. The rest—the non-metal portion—is about 30 percent oxygen and 15 percent silicon, along with smaller amounts of numerous other non-metal elements.
LOOK! SHINY!
For at least a few million years, human beings and their ancestors used tools made from such materials as wood, bone, and rock, to help make their lives a little easier. It didn’t make their lives that much easier: Homo sapiens have been relatively primitive nomadic hunters and gatherers for almost all of their existence. Then, around 10,000 years ago they began discovering ways to work with a “new” material: metal.
The first metals used by humans were the ones that early metalsmiths didn’t have to do very much with to make them usable. These are the native metals—metals that occur in nature in a pure state, or are naturally mixed with other elements in a way that maintains their usable properties. They include copper, tin, lead, silver, and gold.
Someone might have just found nuggets of these metals in a streambed, or in the roots of an unearthed tree, and thought they were attractive. They may have pounded them with stone hammers and found that they could shape them. That could have led to metals being used in jewelry or ornaments, or to the making of metal tools and weapons like axes, knives, and swords—a vast improvement over the old stone tools. All of this eventually led to people actively searching for more metals, the establishment of mines, trading in metals between different peoples, and the birth of a metal industry. However it happened—it happened in numerous locations all over the world.
METALLURGY
Starting around 8,000 years ago, people started discovering that they could alter the metal. They may have discovered it by accident, or perhaps people just got creative, or maybe it was a combination of both. In any case, new processes were developed to alter metals, then to create entirely new ones that didn’t exist in nature at all—with huge improvements in quality. Over the next few thousand years, mining and metalworking became integral to most of the cultures on Earth, and metal became one of the most civilization-changing substances in human history. Each of these new processes involved fire, and it’s likely that experimentation with one led directly to the next. The most important advancements:
- Annealing. This is simply the process of heating metal until it’s cherry red. This restores old, brittle metal to its original malleable state, allowing it to be reworked and prolonging its usability. Annealing can be done at relatively low temperatures (copper can be annealed in a campfire). It was first done sometime around 6000 B.C., somewhere in the Middle East, and possibly in Europe and India around the same time.
- Smelting. In this process, metals are melted into a liquid state, offering for much more freedom to shape them into different forms. Metals were first smelted around 5000 B.C., after the development of more advanced pottery kilns, which can produce much higher heats than could be achieved in simple open fires.
- Alloy Production. This is the process of mixing different metals while they are in a molten state. It began around 3300 B.C. (the beginning of the Bronze Age), with the first production of bronze—a mixture of copper and tin that is much harder and more durable than either of its components.
- Extraction. With further improvements in kiln technology and the subsequent ability to achieve higher temperatures, techniques were developed that allowed for the extraction of metals from ore. It was first done with iron in the Middle East around 1500 B.C.—marking the beginning of the Iron Age.
- Smelting, alloy production, and extraction were practiced by ancient peoples in Europe, Asia, South America, and as far north as Mexico, but not in the rest of North America, or in Australia, until Europeans arrived. These simple processes remain the foundation of what is likely the largest and most successful industry in human history: the metal industry.
IRON
Iron is the most abundant metal on Earth. But like most metals, getting to it is tricky, because it’s very rarely found in a pure state in nature. It most commonly exists in iron oxides—molecules composed of iron and oxygen, which are found mixed with rock in iron ore. To get the iron, you have to get rid of the oxygen and the rock. Here’s the most common process used today:
- Preparation: After being mined, iron ore is crushed into a powder. Huge magnetic drums are then used to separate iron-poor from iron-rich ore. (The iron-rich ore sticks to the drums; the rest falls away.) The iron-rich powder is mixed with clay and made into marble-sized pellets, which are then heat-hardened. That allows for more efficient burning during the next step, smelting.
- Smelting: The pellets are smelted in a furnace along with coke—coal that has been processed into almost pure carbon—and limestone. The intense heat breaks the iron-oxygen bonds in the ore, releasing the oxygen as gas, which bonds with carbon gas being released from the burning coke to form CO2 (carbon dioxide). The CO2 escapes from the top of the furnace, and the iron, now free of the oxygen, melts (at about 2,800°F) and collects at the bottom of the furnace. The limestone also melts and bonds with impurities to form molten waste known as slag. Slag is lighter than iron, and it’s continuously removed from the top of the furnace.
- Result: The product of this process is the iron alloy pig iron. It has a relatively high carbon content of around 5 percent, which makes it very brittle, and pig iron is therefore mostly useless except in the manufacture of other iron alloys, especially steel.
STEEL
Today about 98 percent of pig iron produced worldwide goes into the production of steel, the most widely used metal or metal alloy in history. The process begins by pouring molten pig iron into steel furnaces, where it is treated to remove any remaining impurities, and to lower the carbon content to between 0.1 and 2 percent. That’s one of the chief characteristics of steel: All but a very few of the hundreds of different types of steel contain carbon at these levels. That reduces the brittleness, while increasing strength and hardness. Depending on the type of steel being made, different elements are then added to the mix. Two examples:
- Manganese steel, or mangalloy, is about 13 percent manganese, which results in it being extremely impact-resistant. That makes mangalloy popular for use in mining tools, rock crushing equipment, and armor plating for military vehicles.
- Stainless steel is actually a name for a wide range of steels, but they all have one thing in common: chromium, from about 10 to 30 percent, depending on the type. The chromium on the surface of stainless steel bonds with oxygen in the air to form a layer of chromium-oxide, which is what gives stainless steel its very hard, shiny appearance, and makes it resistant to corrosion. And if it’s damaged or scarred, the chromium re-bonds with oxygen, and a new layer forms—so it’s self-repairing. Stainless steels are used in a wide variety of products, from kitchen utensils to surgical equipment to outdoor sculpture. (It’s also 100% recyclable.)
ALUMINUM
The most common ore used for aluminum production is bauxite, a claylike substance that is around 50 percent alumina—aluminum bonded with oxygen. As with iron, getting to the aluminum means getting rid of the oxygen and the minerals in the ore. The process is much more complicated than iron extraction, and was only developed in the late 1800s. (Aluminum was only identified as a unique element in 1808.) The first part of the system most commonly used today is called the Bayer process, named after Austrian chemist Karl Bayer, who invented it in 1877.
The Bayer Process: Bauxite is mined and crushed, then mixed with water and lye, and heated in tanks. This heat and lye cause the alumina in the ore to dissolve in the water, while impurities sink to the bottom. The alumina-rich water is then siphoned off and filtered to remove further impurities, and then pumped into huge precipitation tanks, where the water is allowed to precipitate away. What remains is a white crystalline powder that is about 99% alumina. The crystals are washed and allowed to dry.
The next step is known as the Hall–Héroult process, named for the two chemists who developed it—independently of one another—in 1886. In this process, the alumina crystals (along with minerals that aid in the breakdown of alumina) are smelted at about 1,760°F in steel vats. But that’s not enough to break the aluminum-oxygen bonds in the alumina; they’re much stronger than iron-oxygen bonds. So a powerful electric current is sent through the molten material—and that causes the bonds to break. The oxygen is released as gas, and is attracted to carbon rods suspended above the molten mix, where it bonds with carbon to form CO2 gas (just like in the iron smelting process). The freed-up aluminum melts and collects at the bottom of the pot. At this point it is 99.8% pure aluminum.
Aluminum is used in a wide variety of applications, in its pure form (aluminum foil is made from nearly pure aluminum), and more commonly in alloys, mixed with elements such as silicon, copper, and zinc. Some are stronger than steel, and have the added benefit of being much lighter. Common uses include in cookware, soft drink cans, and automobile engine blocks.
PLATINUM
Platinum is a shiny, silver-white metal that is very rare and has some unique qualities: It’s one of the densest metals, yet it is very malleable; it is extremely resistant to corrosion by temperature, rust, or exposure to materials such as acids; and it has a very high melting point of 3,215°F (Gold’s melting point is just 1,064°, and iron’s is 1,535°.) Platinum does exist in pure form in nature, but it’s more commonly found mixed with other elements, including oxygen, copper, and nickel. More than 90 percent of the platinum mined in the world today comes from just four sites: three in Russia and one in South Africa. Production is quite complicated.
More than ten tons of ore must be mined to make a single ounce of platinum. A brief description of the process is as follows:
- Ore is mined, crushed to powder, and mixed with water and chemicals. Air is blown through the mix, creating bubbles—to which the tiny platinum particles stick. The bubbles rise to the surface of the tank, creating a soapy froth. The froth is collected, dried, and smelted at temperatures above 2,700°F. The heavier particles—the metals—sink to the bottom of the furnace. Lighter impurities collect on top of the molten metal and are removed. Complicated chemical processes are then used to separate the platinum from any copper, nickel, and other metals still present, until, finally, pure platinum is obtained.
SHINY BITS
- Iron ore is smelted in a blast furnace: Superheated air—up to 2,200°F—is “blasted” into the furnace, causing it to burn much hotter than it otherwise could. A typical blast furnace at a steel mill runs for 24 hours a day, 365 days a weeks, for up to 20 years, before it must be replaced.
- Pure steel is very susceptible to rust. Galvanized steel is steel coated with zinc—which is very resistant to rust.
- A major chemical ingredient in rubies, emeralds and sapphires: aluminum.
- What is most of the extremely rare metal platinum used for? Catalytic converters—the devices on automobiles used to clean exhaust. Platinum is an exceptionally good catalyst: it aids in the conversion of toxic gases in exhaust, such as carbon monoxide, into non-toxic gases.
- It’s a myth that there was no metalworking among Native Americans. Many tribes actually had long traditions of copper-working, especially around the Great Lakes, where the metal was naturally abundant.
- All the platinum mined in history could fit into an average home basement.
 This article is reprinted with permission from Uncle John’s 24 Karat Gold Bathroom Reader. The information miners at the Bathroom Readers’ Institute have unearthed a priceless collection of surprising, amazing, headscratching, and hilarious articles. 24-Karat Gold is chock-full of little-known history, random origins, weird news, celebrity secrets, and urban legends.
This article is reprinted with permission from Uncle John’s 24 Karat Gold Bathroom Reader. The information miners at the Bathroom Readers’ Institute have unearthed a priceless collection of surprising, amazing, headscratching, and hilarious articles. 24-Karat Gold is chock-full of little-known history, random origins, weird news, celebrity secrets, and urban legends.
Since 1987, the Bathroom Readers’ Institute has led the movement to stand up for those who sit down and read in the bathroom (and everywhere else for that matter). With more than 15 million books in print, the Uncle John’s Bathroom Reader series is the longest-running, most popular series of its kind in the world.
If you like Today I Found Out, I guarantee you’ll love the Bathroom Reader Institute’s books, so check them out!
| Share the Knowledge! |
|





“After many millions of years, those first hydrogen and helium atoms collected in clouds of dust and gases so huge”
So this “dust” was made of?
dirty space dust? H, He, and dark matter? But I see your point. Author is trying to compress a few billion years of events into a short non-scientific paragraph, so I’ll allow him some leeway.
The metal in the ring on my finger is older than the planet….well yes, and so is my finger. The calcium, nitrogen, oxygen, iron, carbon, sodium, etc are also from some ancient star. And the hydrogen is even older than that.