Does the Ocean Continually Get Saltier?
 Most sea salts come from water-caused erosion, whereby rivers ultimately carry the dissolved salts to the oceans. Absent a few key processes, the ocean’s salinity would continuously increase; however, there are several mechanisms, called “salt sinks,” that help remove salts from the oceans at pretty much exactly the same rate as they are added.
Most sea salts come from water-caused erosion, whereby rivers ultimately carry the dissolved salts to the oceans. Absent a few key processes, the ocean’s salinity would continuously increase; however, there are several mechanisms, called “salt sinks,” that help remove salts from the oceans at pretty much exactly the same rate as they are added.
One major sink is thanks to the evaporation of water. Once the seawater evaporates, the salt concentration increases. How does that remove salt from the water? Ultimately the water will become supersaturated in certain places and no longer capable of keeping all the salts dissolved, resulting in the formation of evaporite deposits in the sediment that eventually cement into sedimentary rocks.
A second, related sink, uses the wind to spray seawater back onto the land, where the water evaporates, leaving behind salt deposits.
Other sinks rely on chemical processes. For example, lava on the ocean floor will react with dissolved salt ions (like Mg2+), removing them from the water. In addition, certain clays absorb some salts (e.g. Mg2+ and K+), and some hydrogenous minerals, like ferromanganese nodules are also formed by using salts, all resulting in a decrease of ocean salinity.
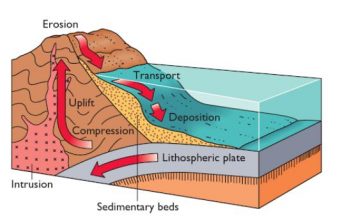 Sea life also help to remove salts from the ocean. Many animals ingest or otherwise extract salts from the water, and this can become incorporated into the organism, such as with a shell (from salts like Si4+ and Ca2+), or it can be excreted (read: pooped). These fall to the ocean floor and become part of the layer of sediment there. Similar to evaporite minerals, these are eventually incorporated into sedimentary rocks.
Sea life also help to remove salts from the ocean. Many animals ingest or otherwise extract salts from the water, and this can become incorporated into the organism, such as with a shell (from salts like Si4+ and Ca2+), or it can be excreted (read: pooped). These fall to the ocean floor and become part of the layer of sediment there. Similar to evaporite minerals, these are eventually incorporated into sedimentary rocks.
Beyond salt sinks, freshwater from rivers, melting ice, and the like also supply a steady stream of comparatively fresh water to the oceans, helping to balance out the loss of water via evaporation.
Together, these inputs and outputs ultimately keep global ocean salinity in a relative state of equilibrium, though there are always regions of the oceans that are more or less salty depending on a variety of factors.
This may all seem somewhat fortuitous (particularly given how important ocean salinity level is to climate), but, in fact, this equilibrium is in no small part because the rate of salt removal from the ocean is directly related to its concentration- higher salinity = higher rates of removal via the aforementioned salt sinks and vice versa.
As a result, for at least the past 1.5 billion years or so the concentration of salts in global seawater has stayed relatively constant at 3.5%.
This, however, has begun to measurably change in the last half century, potentially with disastrous long term consequences if the trend continues. (More on this in the Bonus Facts below.) If you guessed climate change has something to do with the decrease in ocean salinity, you get a gold star.
If you liked this article, you might also enjoy our new popular podcast, The BrainFood Show (iTunes, Spotify, Google Play Music, Feed), as well as:
- The Difference Between Kosher Salt and Table Salt
- What are Smelling Salts and Do They Actually Work?
- Why Iodine is Added to Salt
- Fact or Myth: Sodium Raises Blood Pressure
- Do Cow Farts Really Significantly Contribute to Global Warming?
Bonus Facts:
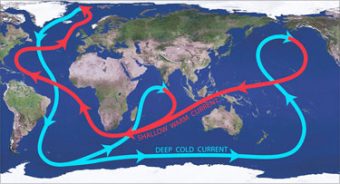 The reason ocean salinity is so important to global climate has to do with the currents in the oceans acting as an enormous “conveyor belt,” moving warm water from the equator and subtropics to the poles, and cooler water from the poles back to the hotter areas (in a process called thermohaline circulation). Since there is more heat stored in the top nine meters of the ocean than in Earth’s entire atmosphere, this movement of heat and cold helps control climate all over the world. Were it to stop or slow significantly, dry areas would become drier and wet areas wetter. This would also result in more extreme temperature ranges in different parts of the world, with some places getting hotter and others cooler. What does this have to do with ocean salinity? Salts play a key role in keeping this conveyor belt moving as saltwater density is one of the main drivers of underwater currents, in a nutshell helping dense, cooled water sink as it gets closer to the poles. However, with melting ice along with higher than normal rainfall currently significantly decreasing ocean salinity around these cooling regions, and certain sub-tropical regions getting even more salty for a variety of reasons, this could potentially negatively impact this “conveyor belt.”
The reason ocean salinity is so important to global climate has to do with the currents in the oceans acting as an enormous “conveyor belt,” moving warm water from the equator and subtropics to the poles, and cooler water from the poles back to the hotter areas (in a process called thermohaline circulation). Since there is more heat stored in the top nine meters of the ocean than in Earth’s entire atmosphere, this movement of heat and cold helps control climate all over the world. Were it to stop or slow significantly, dry areas would become drier and wet areas wetter. This would also result in more extreme temperature ranges in different parts of the world, with some places getting hotter and others cooler. What does this have to do with ocean salinity? Salts play a key role in keeping this conveyor belt moving as saltwater density is one of the main drivers of underwater currents, in a nutshell helping dense, cooled water sink as it gets closer to the poles. However, with melting ice along with higher than normal rainfall currently significantly decreasing ocean salinity around these cooling regions, and certain sub-tropical regions getting even more salty for a variety of reasons, this could potentially negatively impact this “conveyor belt.”- In 2011, NASA and the Comisión Nacional de Actividades Espaciales (CONAE), Argentina’s space agency, with technological assistance from France’s Centre National D’Etudes Spatiales (CNES) and Italy’s Agenzia Spaziale Italiana (ASI), launched a satellite, SAC-D, containing an instrument, Aquarius, used to measure and map global changes in ocean salinity, and to better understand ocean circulation. Among many other things, data from Aquarius seems to indicate that a large plume of freshwater can increase the ultimate intensity of hurricanes. Off the northeast coast of South America, where two large rivers, the Amazon and Orinoco, empty into the Atlantic, at its peak the two rivers “create a plume of low salinity water that . . . covers . . . over 380,000 square miles,” to a depth of just over three feet. Observing Hurricane Katia in 2011, the team learned that the plume apparently prevented Katia from pulling up deep, cold, salty water to the surface (something that is common with hurricanes and is another key factor in regulating global climate). Without it, the warmer surface temperature water contributed to a stronger hurricane, and helped Katia to ultimately become a Category 4. According to the researchers, 68% of Category 5 hurricanes in the Atlantic, at one point, crossed this plume, leading them to opine that ocean salinity plays a key role in cooling off and dampening hurricanes.
- Hurricanes typically die down very quickly after striking land because they need warm water to continue powering themselves; they are in effect gigantic heat engines. They are powered by so much heat that they can release 50-200 exajoules of heat energy per day. This is about the same amount of energy as would be released by detonating 45,000 nuclear bombs per day of the explosive capacity of “Little Boy”, the bomb dropped on Hiroshima. To put it another way, this is about 200 times more energy than human beings currently have the ability to generate if every electrical power plant on Earth was working at 100% capacity for the entire day.
- The U.S. government once tried to develop a way to stop hurricanes from forming, or at the least weaken them. The attempt was known as “Project Stormfury,” specifically focusing on putting silver iodide in the storms, which would freeze water in the outermost bands of rain, hopefully collapsing the inner eye wall and basically stopping the heat engine in its tracks, or at least reducing its power. While it seemed like it worked a bit at the time, in retrospect, it’s thought that their efforts had almost no effect. One seeded hurricane, Hurricane Debbie, initially reduced its intensity by about 30%, but quickly recovered and was as powerful as ever even after a second seeding attempt. It was later discovered that hurricane eye walls cycle, so that 30% drop was probably just part of the cycle and had little to do with the silver iodide. While they didn’t manage to stop a hurricane, in another attempt, a hurricane that would have struck away from highly populated regions, after being seeded, shifted course and struck Savannah, Georgia. Needless to say, seeding hurricanes with silver iodide isn’t something anyone does any more. Numerous other ideas have been proposed to cool the eye, but the simple fact of the matter is that the amount of heat energy being used here is just too much for any known practical solution to work, even considering the billions of dollars of damage annually hurricanes do.
- More to the point, it would be a bad idea to try to stop the hurricanes, even if we could. While tropical storms and hurricanes cause a lot of damage to human settlements, they are actually a critical part of the Earth’s atmospheric circulation system, carrying heat energy from the tropics into colder latitudes, at the same time cooling the upper layers of the ocean over where the storm passes, not just from using the heat energy, but also from churning the water and mixing the upper warm layers with water from the deeper cooler layers of the ocean, as previously mentioned. They also transport massive amounts of water inland to help relieve drought. Besides the global climate effects, it is thought that if we were to stop this from happening, the waters around the equator would continue to collect heat creating even more massive hurricanes, which would be increasingly difficult to stop, possibly even creating a cataclysmic hurricane.
- It is theorized by some researchers, such as professor of meteorology at MIT Kerry Emanuel, that such a cataclysmic hurricane may have been what wiped out the dinosaurs. The theory is that an asteroid strike could have heated parts of the ocean as much as 90 degrees Fahrenheit (50 degrees Celsius) over the normal temperatures. The extra heat energy would have resulted in super-hurricanes the likes of which have never been seen by humans, with wind speeds well over 700 mph (1,130 k/h). It wouldn’t just be the wind speeds that would then cause the death of the dinosaurs, but also the fact that this would have allowed water vapor to be carried up into the Earth’s stratosphere, causing catastrophic climate changes.
- Even without such a super hurricane, it is thought that lesser “super” hurricanes were the norm even just 1-3 thousand years ago. This is based on core samples taken deep inland near the Gulf of Mexico, which indicate that sand from the ocean was regularly carried far further inland than hurricanes today do and with more regularity (about 3-5 times more hurricanes per year than is the average today).
- It’s estimated that if you removed all the salt from the oceans of the world, it could cover every square inch of dry land on Earth with a layer of salt about 500 ft, or around 150 meters, deep.
| Share the Knowledge! |
|





Quote: “…for at least the past 1.5 billion years or so the concentration of salts in global seawater has stayed relatively constant”
And in literally the next paragraph “This, however, has begun to measurably change in the last half century, potentially with disastrous long term consequences if the trend continues.”
My comments are mostly just an exercise in critical thinking: How do we know they’ve been relatively constant over “at least the past 1.5 billion years”? (or so)
Were they *measurably* relatively constant? Of course they weren’t, unless permian amphibians passed on their technology to triassic early dinosaurs and asked them to keep up the good work of measuring ocean salinity? Forgive my sarcasm but sometimes the speculations, guesses and hand-waving that goes into paleo-sciences is akin to writing fairy tales.
And the fluctuations in the past 50 years? have they been greater than the fluctuations in the preceding 1.5 billion years, and how do we know that they will have “potentially disastrous” consequences, given that there may have been more rapid shifts in salinity in the “relatively constant” eras of the past with zero negative effects, possibly even positive ones? What metric would we use to evaluate what a disastrous consequences would be – disastrous for whom? What if the massive Permian extinction event was caused by a sudden spike or decrease in ocean salinity? – would such even be preserved in the fossil record? It seems to me there is a lack of self-criticism in the thinking of sciences advancing such claims. Just my comment. Haters gonna hate, potaters gonna potate.
I agree that the interaction of salts with the water is more complicated than its always been constant. Certainly water moves around, it freezes, it evaporates and the salinity fluctuates as a result of that. It also fluctuates with temperature, and pressure. I am not a geologist and so I do not know who an ancient oceans salinity would be measured. This field is called paleosalinity. I do believe you are correct that they are wrong.
You said: “This may all seem somewhat fortuitous (particularly given how important ocean salinity level is to climate),” – You mean that we have been lucky enough that the Big Bang just happened to move enough cosmic dust to this region, place this rock just the exact right distance from the sun, tilt it at exactly the right degree angle, and then – then – Luck/Chance/chemistry had the time and forethought to set up a salinity balancing mechanism (or several) just for us? Cool! Sure am glad Luck thought that far ahead!
I think it’s widely understood that the amount of salts has changed and has been impacted by many known events such as Messinian Salinity Crisis and events such as the Cretaceous-Paleogene extinction. It would be interesting to know if there are any true studies related to historical salt concentrations as this article is clearly inaccurate.